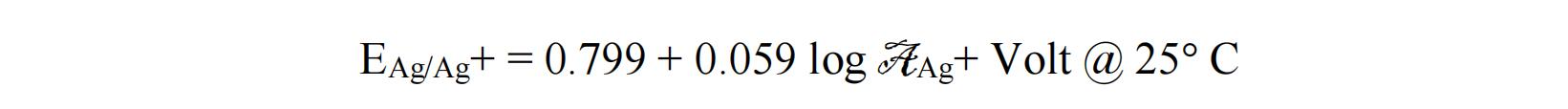Product Details
The silver-silver ion (Ag/Ag+) electrode is a commonly used reference electrode. It is suitable for electrochemical determination of samples in organic solvents.
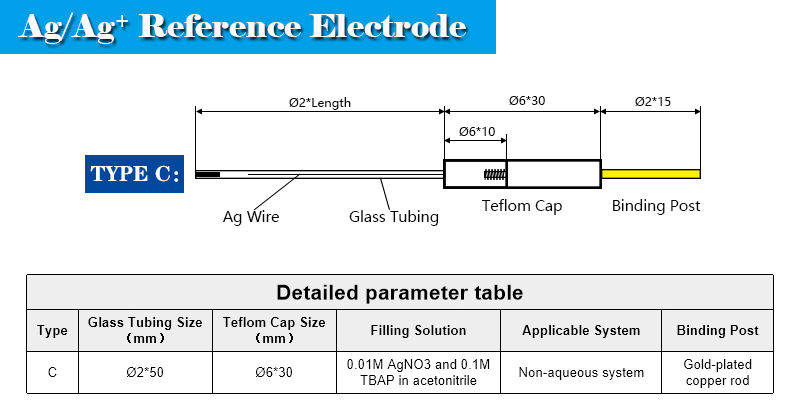
Enclosed is a non-aqueous Ag/Ag+ reference electrode. It consists of a Teflon cap with a Ag wire, a glass tubing with filter core.
You need to fill the compartment (the glass tubing) with Ag+ solution. Dissolve AgNO3 (not include) together with the supporting electrolyte in the organic solvent you work with. The concentration of Ag+ is typically 10 mM. Use a syringe to fill this solution into the glass tubing. The solution level should be no more than 2/3 of the tubing volume. Tap the tubing and make sure that no air bubble is trapped in the bottom of the tubing. Slowly insert the glass tubing into the cap with Ag wire and you obtain a non-aqueous reference electrode.
When you insert the tubing into the cap, the solution in the tubing should not leak at the porous tip. If this does occur, t,refill the Ag+ solution and insert the tubing into the cap again.
The concentration of Ag+ solution can be other values. The potential of the reference electrode versus the Standard Hydrogen Electrode (0.0V) can be calculated from the activity (not concentration) of Ag+ ion according to:
The solution in the compartment is refillable. Please check the solution level in the glass tubing once a while. If the solution level is too low, you should refill the glass tubing with Ag+ solution. To do that, carefully unplug the Teflon cap and follow the instructions given above.
After you fill the reference electrode compartment, you have to make sure not to expose the porous tip to the air. Otherwise the solution in the glass tubing with leak and dry out. The porous tip may also be damaged. When the electrode is not in use, please leave the porous tip in the solvent.
Specification:
tube diameter: 2 mm
effective length: 50 mm
liquid junction core: PTFE filter core
filling solution: 0.01M AgNO3 and 0.1M TBAP in acetonitrile
potential stability: <5 mV
operating temperature: 0-75 degrees Celsius
Different research systems can choose different reference electrodes:
Neutral system------saturated calomel electrode or Ag/AgCl reference electrode
Alkaline system-----Hg/HgO reference electrode
Acidic system-------Hg/Hg2SO4 reference electrode
Non-aqueous system--Non aqueous Ag+ reference electrode
1.This is a customized item, we can't offer any refund or exchange. If you have any questions before purchasing, please feel free to communicate with us.
2.Due to shipping safety considerations, all reference electrodes are not filled with solution. Before use, the user needs to prepare an internal solution to fill it into the electrode.
Product Includes: 1 * Ag/Ag+ reference electrode with gold-plated pin 1 * syringe 1 * Fine needle 1 * cap 1 * fluorine rubber O-ring
NEED HELP? CONTACT US
Call Toll-Free +1 (800) 972-7086
Email Us:
- Shipping
- Standard Shipping: 5-7 Business days
- DHL/Fedex Express Shipping: 2-5 Business days
PAYMENT
PRODUCT SHOW
Here are some photos, you can find the details from them, if you need more information, you can talk with us, we have many experts in dekresearch, and we are very happy to give you some support in your items!
We devotes itself to providing laboratory instrumentation that enables simultaneous in-situ measurements of a number of signals (electrochemical, optical, thermal and other) on thin film and membrane materials. we have already gained so much experience in the field of electrochemical trading and our final goal is to offer our customers various kinds of laboratory instrumentation and at best price. We are gradually becoming one of the top instrumentation online shop throughout the world and we do hope we could make you outstanding by providing the real good stuff you want. Enjoy your shopping with us and have a nice day.

 United Kingdom (GBP)
United Kingdom (GBP) Canada (CAD)
Canada (CAD) Australia (AUD)
Australia (AUD) Singapore (SGD)
Singapore (SGD) New Zealand (NZD)
New Zealand (NZD) Ireland (EUR)
Ireland (EUR) India (INR)
India (INR)